Efficiently smuggling drugs into cells

Fluorescent soft beads passing through the narrowing channels of a biochip. Photo: MPZPM, Salvatore Girardo
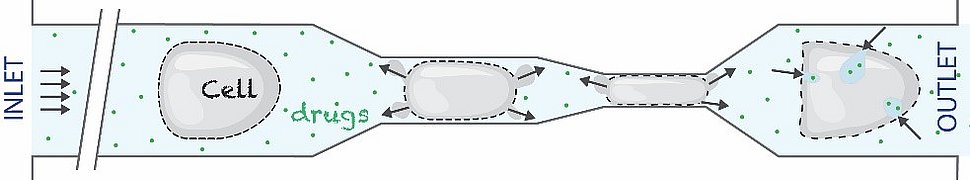
The cells are stretched by passing through the nanochip. This creates pores in their cell membrane, allowing molecules to pass through and get inside the cells. Graphic: Ruchi Goswami
A new, patented method called Progressive Mechanoporation makes it possible to mechanically disrupt the membranes of cells for a short time period and let drugs or genes inside cells. In this way, researchers can test new therapies more easily than before.
Modern vaccines such as those against Sars-CoV-2 use tiny lipid spheres to transport genetic information into cells and let the body build up an immune defense against the virus. A team of scientists from Erlangen, Dresden, and London has now developed a completely new method to very efficiently deliver not only genes but also drugs and other substances into cells. The researchers from the Max-Planck-Zentrum für Physik und Medizin (MPZPM) in Erlangen, the Technical University of Dresden, and The Institute of Cancer Research in London have named the method Progressive Mechanoporation and have now published it in the scientific journal "Lab on a Chip". They have also filed a patent.
Ruchi Goswami and Alena Uvizl were part of a team of scientists led by Salvatore Girardo (Erlangen) and Jörg Mansfeld (Dresden/London) who have developed the Progressive Mechanoporation. They have built a special polymer biochip that contains a series of microchannels. Each microchannel is narrower than the previous one, finally reaching a size more than ten times thinner than a human hair. The scientists pass the cells through these channels, causing the cells to stretch more and more. The stretching creates pores in their cell membrane, allowing molecules to pass through these pores and get inside the cells. Once the cells have passed through the channels, the pores close again. The scientists demonstrated that Progressive Mechanoporation can deliver even very large proteins inside the cells. As a proof of concept the scientists used antibodies and CRISPR/Cas9, the genetic scissors whose discovery led to a last year’s Nobel Prize.
Potentially a new routine procedure for hospitals
"The big advantage of our method is that we can pass up to 10,000 cells per second through the chip," explains Salvatore Girardo, leader of the technology development and service group Lab-on-a-Chip at the MPZPM. At the same time, the method is very gentle. Compared to other techniques, very few cells get damaged.
The Progressive Mechanoporation method could be used in drug development and allow pharmaceutical companies to efficiently test new molecule candidates. In addition, the process can be easily automated. Jörg Mansfeld, a research group leader at the Biotechnology Center (BIOTEC) of TU Dresden and at The Institute of Cancer Research, London, adds: "I can envision that in the future, hospitals will be able to routinely examine and even treat patients' cells using Progressive Mechanoporation."
Original publication:
Alena Uvizl, Ruchi Goswami, Shanil Durgeshkumar Gandhi, Martina Augsburg, Frank Buchholz, Jochen Guck, Jörg Mansfeld and Salvatore Girardo: Efficient and gentle delivery of molecules into cells with different elasticity via progressive mechanoporation. Lab Chip, 2021, Advance Article. doi: 10.1039/d0lc01224f
Media inquiries:
Max-Planck-Zentrum für Physik und Medizin
Lothar Kuhn
Tel: +49 9131 7133-825
presse@mpzpm.de
Center for Molecular and Cellular Bioengineering, TU Dresden
Dr. Magdalena Gonciarz
Tel.: +49 351 458-82065
cmcb_press@tu-dresden.de
Contact
Edda Fischer
Head of Communication and Marketing
Telefon: 09131 7133 805
MPLpresse@mpl.mpg.de






